CNRS thematic school 2023
"Methods and Tools for the Electron Density Analysis to Rationalize the Molecular Interactions (MoDerm)"
Sorbonne University (Pierre et Marie Campus)
from Monday September 4, 2023 2 p.m. to Thursday September 7 5 p.m.

The analyses of the electron density allow to rationalize the quantum computations in chemical terms such as bonds, lone pairs, electrophilicity... These methods complement other approaches, such as the orbital analyses and provide a more complete picture of molecular interactions that would otherwise be difficult to obtain experimentally.
The MoDerm 2023 school aims at introducing non-expert researchers to the main methods and tools of the electron density analysis. The school will provide an opportunity to learn about these specialized methods and tools from scratch and how they can be used to gain insights into the molecular properties and reactions. The school will offer also the oppurtunity for more avanced colleagues to develop their expertise on these methods.
This school will present two main families of analysis of interactions in the molecule. Prior knowledge of quantum chemistry and its concrete application through the use of standard software is strongly recommended.
1) The family of so-called "Quantum Chemical Topology" electron density analyses:
The atoms in molecules theory (QTAIM, R. Bader Chemical Reviews, 1991) which analyzes a molecule in terms of atoms and bond paths, directly linked to the usual representations of chemists,
The topological analysis of the electron localization function (ELF, B Silvi and A Savin Nature, 1994) which decomposes the electron cloud into core electrons, bonding and non-bonding pairs,
The analysis of non-covalent interactions. First, that of the molecular electrostatic potential (MESP, J.S. Murray and P. Politzer WIREs Comput Mol Sci 2011) which is a very robust alternative for determining the best sites of interactions between molecules and that of Non-Covalent Interactions (NCI , Erin R. Johnson et al. J. Am. Chem. Soc. 2010).
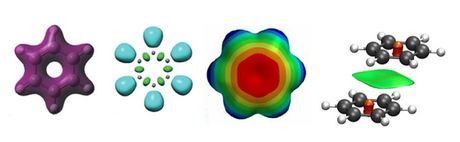
Isosurfaces (from left to right) of electron density, ELF function, molecular electrostatic potential
and non-covalent interactions of benzene.
2) The family of descriptors of the reactivity of the Conceptual Density Functional Theory (cDFT, R. G. Parr and W. Yang 1994): starting from the electron density, one evaluates which sites are likely to undergo attacks by electrophiles, nucleophiles and the speed of these attacks.
 Loading...
Loading...